Reducing Implant Infections in Veterinary Orthopedic Surgery: The Power of Antimicrobial Coating
Implant-associated infections are among the most serious complications in veterinary orthopedic surgery, particularly in procedures like Tibial Plateau Leveling Osteotomy (TPLO). These infections can delay healing, increase the need for revision surgery, and place a financial and emotional burden on clients. As antimicrobial resistance continues to rise, preventive strategies that do not rely solely on systemic antibiotics are critical.
One such solution is HyProtect™, a silver-based antimicrobial coating used on veterinary orthopedic implants. Backed by clinical data, this innovation is helping veterinary surgeons reduce infection risk while maintaining their current surgical protocols.
The Clinical Challenge: Implant-Associated Infections in TPLO Procedures
Surgical site infections (SSIs) and periprosthetic infections remain an ongoing concern, with published reports citing TPLO infection rates as high as 14.3%1. These complications may require implant removal and additional procedures, extending recovery time and increasing overall treatment costs.
For surgeons committed to improving outcomes, integrating preventive tools like antimicrobial-coated implants offers measurable clinical advantages.
HyProtect™: Silver-Based Antimicrobial Coating for Orthopedic Implants
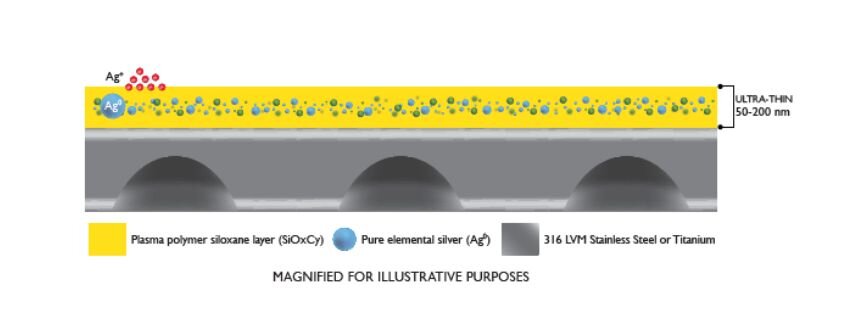
HyProtect™ is an ultra-thin, biocompatible antimicrobial coating applied to orthopedic implants such as the BioMedtrix TPLO Curve and MultiPurpose Bone Plate (MPP). The coating is created using Physical and Chemical Vapor Deposition (PVD + CVD) processes, embedding pure elemental silver (Ag⁰) into a durable plasma polymer siloxane layer.
Once implanted, the coating releases silver ions (Ag⁺) that interfere with bacterial metabolism, DNA replication, and biofilm formation. This process provides sustained antimicrobial activity for at least 100 days postoperatively.
Clinical Evaluation: Prospective Data Supports Efficacy
An ongoing study was developed to understand the potential improvement of the HyProtect antimicrobial coating for each of BioMedtrix’s TPLO Curve and MultiPurpose Bone Plate. Data was collected across 90 clinical sites.
All patients included in the study presented with ruptured cranial cruciate ligaments and were candidates for the TPLO procedure. The same plate and screw designs were used on all patients within each study, in addition to the same surgical technique and postoperative recovery regime. Assessment of patient condition was confirmed at a minimum of 8-12 weeks post-operatively. Owners were advised to contact the practice if any wound or implant issues arose before that time period. Further observations were made to verify that there were no complications or side effects from the coating.
Study Results
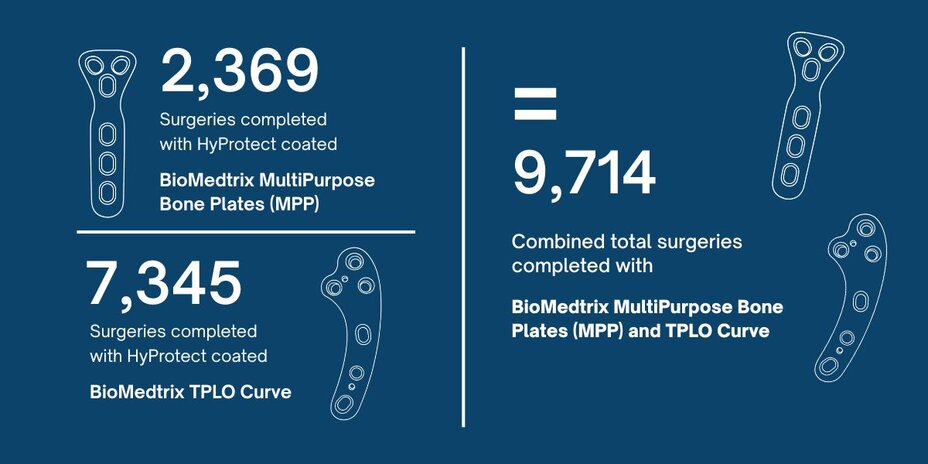
As of February 2019, a total of 7,345 surgeries had been completed using the HyProtect coated BioMedtrix TPLO Curve, and 2,369 surgeries had been completed using the HyProtect coated BioMedtrix MultiPurpose Bone Plates (MPP).
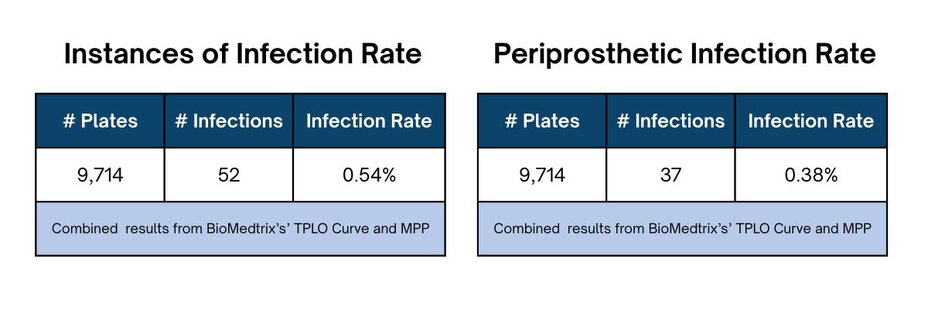
Among the 7,345 HyProtect coated BioMedtrix TPLO Curve surgeries, 40 of these cases have reported instances of infection. Of the 40 reported infections with the BioMedtrix TPLO Curve, 13 were reported to be surgical site infections (SSI), not periprosthetic in origin.
Among the 2,369 HyProtect coated BioMedtrix MultiPurpose Bone Plates (MPP) surgeries, 12 of these cases have reported instances of infection. Of the 12 reported infections with HyProtect coated BioMedtrix MultiPurpose Bone Plates (MPP), 2 were reported to be surgical site infections (SSI), not periprosthetic in origin.
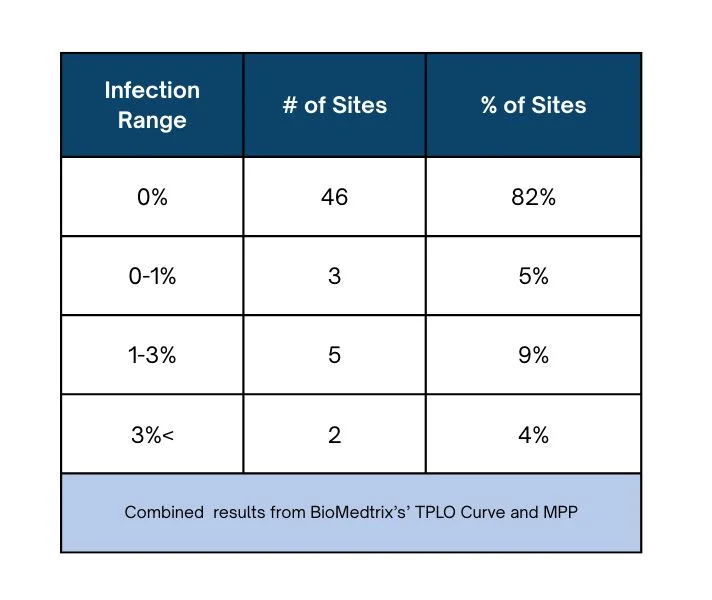
Periprosthetic infection rate distribution
Of the 90 total sites polled for data, 56 sites responded, accounting for 62% of active users of the HyProtect Bone Plates. The periprosthetic infection rate distribution among these 56 responding sites.
These findings highlight the significant reduction in implant-related infection risk and support the broader adoption of silver-coated implants in veterinary practice. This study resulted in a clear reduction of infection rates in patients to only 0.38%.
Why Surgeons are Choosing HyProtect?
Peace of Mind: Continuous silver ion release helps prevent postoperative periprosthetic infection for at least 100 days.
Clinically Proven: Trusted in human and veterinary medicine for over a decade.
Time-Saving: Pre-sterilized implants help streamline and reduce prep time.
Broad-Spectrum Efficacy: Effective against gram-positive, gram-negative, and mult-resistant bacteria.
Biocompatible: Safe for soft tissue and bone healing environments.
Non-Antibiotic Dependent: Limits resistance development and avoids unnecessary systemic antibiotic use.
How it Works? Sustained Ion Release
HyProtect coating forms an ultra-thin (50–200 nm) barrier composed of embedded silver particles that continuously release Ag⁺ ions. These ions:
- Disrupt bacterial cell walls
- Interfere with energy production and DNA replication
- Inhibit biofilm formation on implant surfaces
This mode of action ensures an antimicrobial effect precisely where it’s needed — on the implant surface during the critical early phases of healing.
Clinical Takeaway
By using implants coated with HyProtect, veterinary surgeons gain a scientifically validated tool for infection prevention. It supports better patient outcomes, fewer complications, and reduced need for antibiotics or revision procedures — all while maintaining surgical efficiency and providing better patient outcomes..
Explore the full range of HyProtect™ antimicrobial-coated implants at https://go.movora.com/HyProtect or contact us for more information.
*The veterinary information provided is the result of an independent clinical evaluation performed by BioMedtrix A Movora Company.
References:
1: DiFilippo, L. (2025) Comparison of Surgical Site Infection (SSI) Rates in Dogs Undergoing Tibial Plateau Leveling Osteotomy (TPLO) Using Perioperative Versus Peri- and Postoperative Antimicrobial Prophylaxis. Vet. Sci. 2025, 12(3), 258; https://doi.org/10.3390/vetsci12030258

